
Basic information on electrochemical: coagulation (EC) and oxidation (EO). Electrocoagulation (EC) and Electrooxidation (EO) technologies
Electrocoagulation can be defined as an electrochemical process in which, from compounds resulting from the dissolution of an anode (sacrificial anode), the colloidal matter in wastewater is gathered together, thus enabling its separation through conventional techniques.
Electrooxidation is one of the most important AOPs (Advanced Oxidation Processes) which yield fast oxidation of organics. This process rely, mainly, on the hydroxil radical (.OH) formation, that it’s highly reactive radical which can rapidly degrade anthropogenic organic species, either toxic or refractory that biological treatments can’t treat.
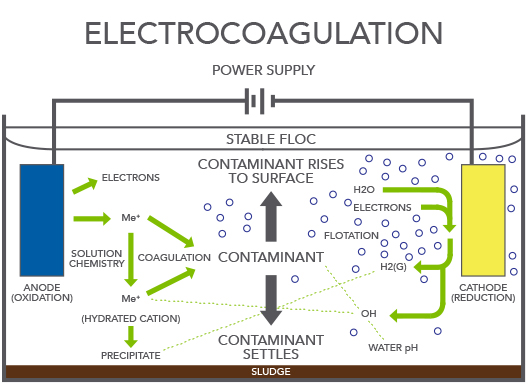
Electrocoagulation is an electrochemical process with the same basis as conventional coagulation. The main innovation is that the coagulant is generated in situ by the anode electrolytic oxidation (iron or aluminum) which is sacrificed, so it is not necessary to add other salts.
The electrocoagulation process is very similar to normal chemical-physical coagulation – the difference is that it uses electrical energy. Both processes aim to destabilize the colloids contained in a quantity of water, but they differ in the method used to add the reagent: in conventional coagulation, the reagent is added as a salt, whereas in electrocoagulation, it is generated from a metal. Electrocoagulation can be defined as an electrochemical process during which the colloidal matter present in a quantity of wastewater is gathered together using compounds resulting from the dissolving of an anode in order to allow the colloids to be separated from the wastewater using conventional techniques. Because they dissolve, the anodes disappear during the course of the treatment, until the moment is reached when they need to be replaced. This means that they are ‘sacrificial anodes’.
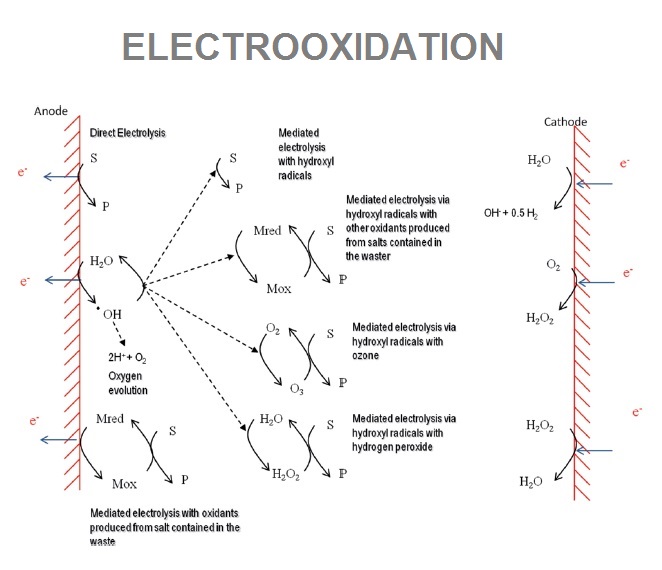
Electrooxidation is an electrochemical process known too as Electrochemical Advanced Oxidation Process (EAOP), where using determinates electrodes (non sacrificial anodes) the treatment produces hydroxil radicals (high oxidizing agents) that can remove non-biodegrabale compounds and even improve contamination biological removal. These processes are divided in two groups depending on the place where predominant mechanisms that explain the overall oxidation develop: surface of the electrodes (anodic oxidation) or bulk of the electrochemical cell. Anodic materials that we use: Boron Doped Diamond (BDD), Graphite and Mixed Metal Oxide (MMO) coated Titanium.
You can find much more scientific-technical information on Internet.
Basic Information: PROELEC EC and EO
PROELEC executes a controlled electrochemical process that destroys toxic compounds. In almost all cases, PROELEC EC improves the chemical-physical traditional treatment, so that in addition to flocculate and coagulate, PROELEC EC is also executing a partial oxidation of the biodegradable contamination and a partial oxidation of the non-biodegradable contamination. In fact, depending on how we proceed and depending on the quality of the water to treat, we can oxidize soluble COD in yields of up to 95% or even more. PROELEC EO is the definitive solution to rapidly degrade recalcitrant organics such as aromatic, chlorinated and phenolic compounds. It can be a very good complement of biological treatment or, even, an alternative in some cases where biological oxidation can not be applied.


